Duchenne muscular dystrophy punnett square, a powerful tool in understanding the inheritance of this devastating neuromuscular disorder, provides invaluable insights into the genetic basis of the disease. This article delves into the intricate interplay of genes, alleles, and inheritance patterns, offering a comprehensive analysis of DMD transmission and its implications for affected families.
The X-linked recessive nature of DMD, coupled with the complexities of Punnett square analysis, raises fundamental questions about the probability of inheriting this debilitating condition. We will explore these concepts, examining the phenotypic expression of DMD and its correlation with genetic mutations.
Genetic Inheritance
Duchenne muscular dystrophy (DMD) is an X-linked recessive genetic disorder caused by mutations in the dystrophin gene located on the X chromosome. The dystrophin gene provides instructions for producing the dystrophin protein, which is essential for the proper function of muscle cells.
Mutations in this gene lead to a deficiency or absence of functional dystrophin protein, resulting in progressive muscle weakness and degeneration.The X-linked recessive inheritance pattern of DMD means that the disorder is primarily passed down from mothers to sons. Females have two X chromosomes, while males have one X chromosome and one Y chromosome.
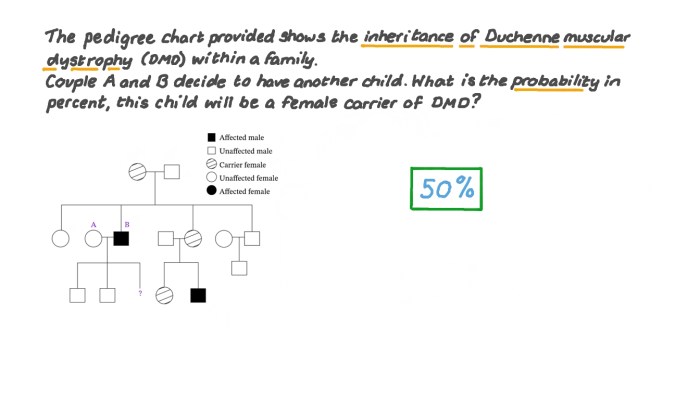
If a female carries a mutated dystrophin gene on one of her X chromosomes, she is considered a carrier. Carrier females typically do not exhibit symptoms of DMD, but they can pass the mutated gene to their children. Males, on the other hand, have only one X chromosome, so if they inherit the mutated dystrophin gene from their mother, they will develop DMD.
Punnett Square Analysis
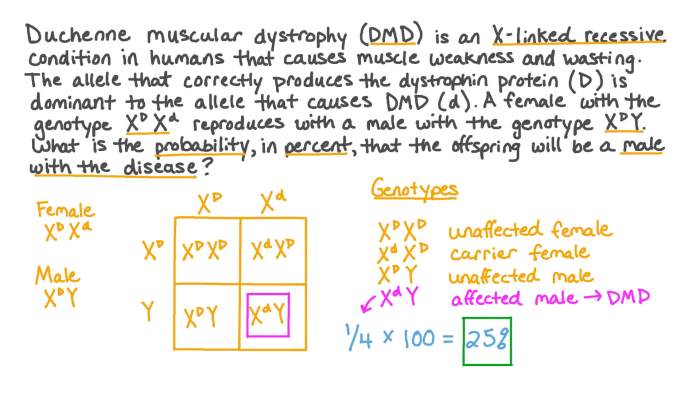
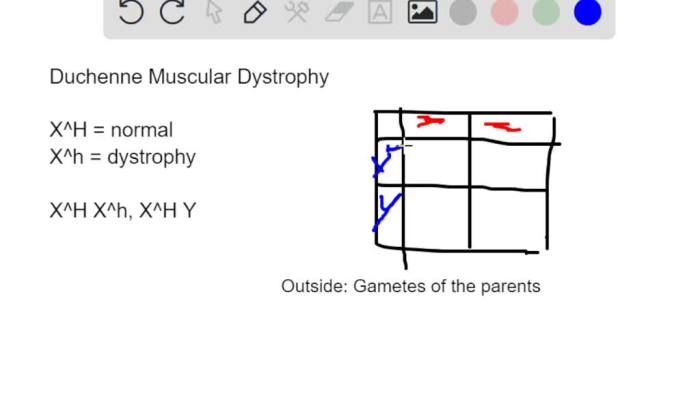
A Punnett square is a diagram used to predict the probability of inheriting a particular genetic trait based on the genotypes of the parents. In the case of DMD, a Punnett square can be used to determine the likelihood of a child inheriting the disorder from affected and unaffected parents.Consider
a family where the mother is a carrier of DMD (X DX d) and the father is unaffected (X DY). The Punnett square below shows the possible genotypes of their offspring:| X D| X d||—|—|| X DX D| X DX d|| X DY | X dY |As you can see from the Punnett square, there is a 50% chance that a son will inherit DMD (X dY) and a 50% chance that he will be unaffected (X DY).
All daughters will be carriers (X DX d).
Phenotypic Expression, Duchenne muscular dystrophy punnett square
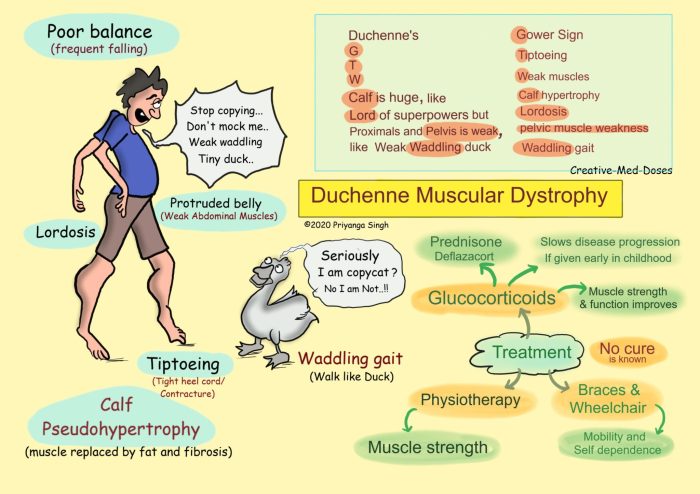
The clinical manifestations of DMD vary widely depending on the severity of the dystrophin gene mutation. The most common symptom is progressive muscle weakness, which typically begins in the lower limbs and gradually spreads to the upper body. As the disease progresses, individuals with DMD may experience difficulty walking, running, and climbing stairs.
They may also develop contractures (shortening of muscles and tendons), which can further limit their mobility.In addition to muscle weakness, DMD can also affect the heart and respiratory system. Cardiac involvement can range from mild arrhythmias to severe cardiomyopathy, which can lead to heart failure.
Respiratory issues can include respiratory infections, sleep apnea, and restrictive lung disease.
Genetic Testing
Genetic testing is essential for the accurate diagnosis of DMD. There are several methods available for genetic testing, including:
-
-*DNA sequencing
This test analyzes the DNA sequence of the dystrophin gene to identify mutations.
-*Deletion/duplication analysis
This test looks for large deletions or duplications in the dystrophin gene.
Genetic testing can be performed on blood or muscle samples. It is important to note that genetic testing can also be used to identify female carriers of DMD.
Treatment and Management
There is currently no cure for DMD, but there are a number of treatments available to manage the symptoms and improve quality of life. These treatments include:
-
-*Corticosteroids
These medications can help to slow the progression of muscle weakness.
-*Gene therapy
This experimental treatment involves introducing a functional copy of the dystrophin gene into muscle cells.
-*Supportive care
This includes physical therapy, occupational therapy, and respiratory support to help manage the symptoms of DMD.
The challenges and limitations of DMD treatment include:
-
-*The progressive nature of the disease
DMD is a progressive disorder, meaning that the symptoms will worsen over time.
-*The lack of a cure
There is currently no cure for DMD, so treatment is focused on managing the symptoms and improving quality of life.
-*The high cost of treatment
The treatments for DMD can be expensive, which can be a significant financial burden for families.
Questions Often Asked: Duchenne Muscular Dystrophy Punnett Square
What is the role of the DMD gene in Duchenne muscular dystrophy?
The DMD gene provides instructions for producing dystrophin, a protein essential for muscle function. Mutations in this gene disrupt dystrophin production, leading to muscle weakness and progressive degeneration.
How does the X-linked recessive inheritance pattern affect DMD transmission?
DMD is located on the X chromosome, which males have one copy of and females have two. Males with a mutated DMD gene on their single X chromosome will develop the disease, while females with a mutated gene on one X chromosome will be carriers.
What are the clinical manifestations of Duchenne muscular dystrophy?
DMD typically presents in early childhood with progressive muscle weakness, particularly in the legs and arms. Other symptoms include cardiac involvement, respiratory issues, and cognitive difficulties.