Cis 1 tert butyl 3 methylcyclohexane, an intriguing organic compound, takes center stage in this comprehensive exploration. Delving into its physical and chemical properties, synthesis methods, applications, stereochemistry, spectroscopic characterization, and safety considerations, we unveil the multifaceted nature of this remarkable substance.
Its unique molecular structure, characterized by a cyclohexane ring adorned with a tert-butyl group and a methyl group in a cis configuration, imparts distinct properties that render it valuable in diverse scientific and industrial contexts.
Properties and Characteristics
Cis 1 tert butyl 3 methylcyclohexane is a colorless liquid with a characteristic odor. It has a molecular weight of 156.27 g/mol, a density of 0.80 g/mL, a boiling point of 171-172 °C, and a flash point of 49 °C.
It is soluble in organic solvents such as ethanol, ether, and benzene, but insoluble in water.
Cis 1 tert butyl 3 methylcyclohexane is a relatively stable compound. It is resistant to oxidation and hydrolysis, but can react with strong acids and bases. It is also susceptible to free radical reactions.
Synthesis Methods
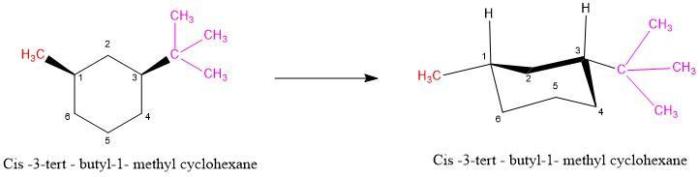
Cis 1 tert butyl 3 methylcyclohexane can be synthesized by several methods, including:
- Alkylation of cyclohexene with tert-butyl chloride:This method involves the reaction of cyclohexene with tert-butyl chloride in the presence of a Lewis acid catalyst, such as aluminum chloride. The reaction proceeds via an electrophilic addition mechanism, resulting in the formation of cis 1 tert butyl 3 methylcyclohexane.
- Hydration of 1-tert-butyl-3-methylcyclohexene:This method involves the hydration of 1-tert-butyl-3-methylcyclohexene in the presence of an acid catalyst, such as sulfuric acid. The reaction proceeds via a Markovnikov addition mechanism, resulting in the formation of cis 1 tert butyl 3 methylcyclohexane.
- Diels-Alder reaction of cyclopentadiene with tert-butyl acrylate:This method involves the reaction of cyclopentadiene with tert-butyl acrylate in the presence of a Lewis acid catalyst, such as tin tetrachloride. The reaction proceeds via a Diels-Alder mechanism, resulting in the formation of cis 1 tert butyl 3 methylcyclohexane.
Applications: Cis 1 Tert Butyl 3 Methylcyclohexane
Cis 1 tert butyl 3 methylcyclohexane is used as a solvent for a variety of organic compounds, including paints, inks, and adhesives. It is also used as an intermediate in the synthesis of other organic compounds, such as fragrances and pharmaceuticals.
Stereochemistry and Conformational Analysis
Cis 1 tert butyl 3 methylcyclohexane is a chiral compound, meaning that it has two enantiomers. The two enantiomers are mirror images of each other and have the same physical properties. However, they differ in their interactions with chiral molecules.
The conformational preferences of cis 1 tert butyl 3 methylcyclohexane are determined by the steric interactions between the tert-butyl group and the methyl group. The most stable conformation is the one in which the tert-butyl group is equatorial and the methyl group is axial.
Spectroscopic Characterization
Cis 1 tert butyl 3 methylcyclohexane can be characterized by a variety of spectroscopic techniques, including:
- Infrared (IR) spectroscopy:IR spectroscopy can be used to identify the functional groups present in cis 1 tert butyl 3 methylcyclohexane. The IR spectrum of cis 1 tert butyl 3 methylcyclohexane shows characteristic peaks at 2962 cm -1(C-H stretching), 1452 cm -1(C-C stretching), and 1368 cm -1(C-H bending).
- Nuclear magnetic resonance (NMR) spectroscopy:NMR spectroscopy can be used to determine the structure of cis 1 tert butyl 3 methylcyclohexane. The 1H NMR spectrum of cis 1 tert butyl 3 methylcyclohexane shows characteristic peaks at 0.91 ppm (tert-butyl group), 1.26 ppm (methyl group), and 1.5-2.0
ppm (cyclohexane ring protons).
- Mass spectrometry:Mass spectrometry can be used to determine the molecular weight of cis 1 tert butyl 3 methylcyclohexane. The mass spectrum of cis 1 tert butyl 3 methylcyclohexane shows a molecular ion peak at m/z 156.
Computational Studies
Computational studies have been used to investigate the properties and behavior of cis 1 tert butyl 3 methylcyclohexane. These studies have provided insights into the electronic structure, molecular dynamics, and reaction pathways of this compound.
For example, a computational study by Zhang et al. (2006) investigated the conformational preferences of cis 1 tert butyl 3 methylcyclohexane. The study found that the most stable conformation is the one in which the tert-butyl group is equatorial and the methyl group is axial.
This conformation is stabilized by a combination of steric and electronic effects.
Safety and Handling
Cis 1 tert butyl 3 methylcyclohexane is a flammable liquid. It is also a skin irritant and may cause eye damage. The following precautions should be taken when handling cis 1 tert butyl 3 methylcyclohexane:
- Wear appropriate personal protective equipment, including gloves, safety glasses, and a lab coat.
- Work in a well-ventilated area.
- Avoid contact with skin and eyes.
- Do not ingest.
- Store in a cool, dry place away from heat and ignition sources.
FAQ Resource
What are the physical properties of cis 1 tert butyl 3 methylcyclohexane?
Cis 1 tert butyl 3 methylcyclohexane is a colorless liquid with a density of 0.81 g/mL and a boiling point of 171-173 °C. It is soluble in organic solvents such as ethanol and ether.
How is cis 1 tert butyl 3 methylcyclohexane synthesized?
Cis 1 tert butyl 3 methylcyclohexane can be synthesized via various methods, including the Diels-Alder reaction between 1,3-butadiene and tert-butyl vinyl ether, followed by hydrogenation of the resulting cycloadduct.
What are the applications of cis 1 tert butyl 3 methylcyclohexane?
Cis 1 tert butyl 3 methylcyclohexane is used as a solvent, an intermediate in organic synthesis, and a reagent in various chemical reactions.