Bohr model of mercury element – Delve into the fascinating world of the Bohr model of mercury, a pioneering theory that revolutionized our understanding of atomic structure. This model, proposed by Niels Bohr, has played a pivotal role in explaining the behavior and properties of this enigmatic element.
The Bohr model depicts mercury as a miniature solar system, with a central nucleus orbited by electrons arranged in discrete energy levels. Each energy level corresponds to a specific amount of energy, and electrons can transition between these levels by absorbing or emitting photons.
Historical Significance of Bohr’s Model for Mercury: Bohr Model Of Mercury Element
The Bohr model, developed by Niels Bohr in 1913, revolutionized our understanding of atomic structure. It introduced the concept of quantized energy levels, where electrons occupy specific orbits around the nucleus. This model was crucial in explaining the behavior of the mercury element, which exhibits unique spectral lines due to its electronic transitions.
Bohr’s Model and Mercury’s Spectral Lines
The Bohr model successfully explained the emission and absorption spectra of mercury. According to the model, electrons in mercury atoms can occupy discrete energy levels. When an electron transitions from a higher energy level to a lower one, it emits a photon of light with a specific wavelength.
Conversely, when an electron absorbs a photon, it jumps to a higher energy level.The Bohr model predicted the wavelengths of the spectral lines observed in mercury’s spectrum, providing strong evidence for the quantized nature of atomic energy levels. This discovery paved the way for further advancements in atomic physics and laid the foundation for quantum mechanics.
The Bohr model of the mercury element, with its distinct electron configurations, provides valuable insights into the atom’s behavior. To delve deeper into atomic structures and their dating implications, refer to the atomic dating game answer key . This comprehensive resource offers a thorough understanding of atomic dating techniques and their applications in unraveling the mysteries of the past.
By examining the Bohr model of the mercury element alongside the principles of atomic dating, we gain a more comprehensive perspective on the fascinating world of atomic structures and their historical significance.
Key Features of Bohr’s Model as Applied to Mercury
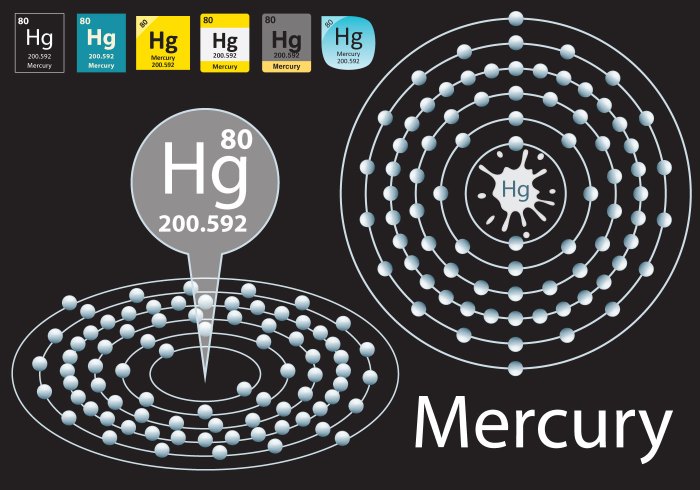
The Bohr model of mercury is a simplified representation of the atom’s structure, which describes the arrangement of electrons in specific energy levels around the nucleus.
The model consists of the following key features:
Energy Levels and Electron Arrangement, Bohr model of mercury element
The Bohr model for mercury has six energy levels, labeled K, L, M, N, O, and P, starting from the innermost level.
- The K level can hold up to 2 electrons.
- The L level can hold up to 8 electrons.
- The M level can hold up to 18 electrons.
- The N level can hold up to 32 electrons.
- The O level can hold up to 50 electrons.
- The P level can hold up to 72 electrons.
In the case of mercury, the electrons are arranged as follows:
- K: 2 electrons
- L: 8 electrons
- M: 18 electrons
- N: 32 electrons
- O: 18 electrons
- P: 2 electrons
Chemical Properties
The arrangement of electrons in the energy levels contributes to the chemical properties of mercury.
Mercury is a transition metal with a silvery-white appearance. It is a poor conductor of heat and electricity, and it has a high density.
Mercury’s chemical properties are primarily due to the presence of the two electrons in the outermost P level.
These electrons are loosely bound to the nucleus, making mercury a relatively reactive element.
Mercury can form a variety of compounds, including mercury(II) chloride (HgCl2) and mercury(II) oxide (HgO).
Limitations and Refinements of Bohr’s Model for Mercury

Bohr’s model, while groundbreaking, had limitations in explaining certain aspects of mercury’s behavior. One key limitation was its inability to account for the element’s magnetic properties.
Subsequent models, particularly the quantum mechanical model, refined and extended Bohr’s model, providing a more accurate description of mercury’s electronic structure. These models incorporated concepts such as electron spin and the Pauli exclusion principle, which allowed for a more complete understanding of the element’s magnetic properties and other aspects of its behavior.
Magnetic Properties and Refinements
- Bohr’s model did not consider electron spin, which plays a crucial role in determining magnetic properties.
- Quantum mechanical models introduced the concept of electron spin, explaining mercury’s paramagnetic behavior.
- The Pauli exclusion principle further refined the model by limiting the number of electrons in each energy level and spin state, leading to a more accurate description of the element’s electronic configuration.
Applications of Bohr’s Model for Mercury in Various Fields

Bohr’s model has served as a cornerstone for understanding the behavior of mercury in diverse scientific disciplines, ranging from chemistry and materials science to astrophysics.
Chemistry
In chemistry, the Bohr model has been instrumental in elucidating the electronic structure of mercury atoms and molecules. It has provided insights into the chemical bonding and reactivity of mercury compounds, aiding in the development of new materials and pharmaceuticals.
Materials Science
The Bohr model has found applications in materials science, particularly in the study of semiconductors and superconductors. By understanding the electronic configurations of mercury atoms within these materials, scientists have been able to tailor their properties for specific applications, such as high-performance electronics and energy-efficient devices.
Astrophysics
In astrophysics, the Bohr model has been used to explain the spectral lines observed in the light emitted by mercury vapor in stars and interstellar gas clouds. These spectral lines provide valuable information about the temperature, density, and chemical composition of these celestial objects, helping astronomers understand the evolution and behavior of the universe.
Mercury’s Position in the Periodic Table and Its Relationship to the Bohr Model
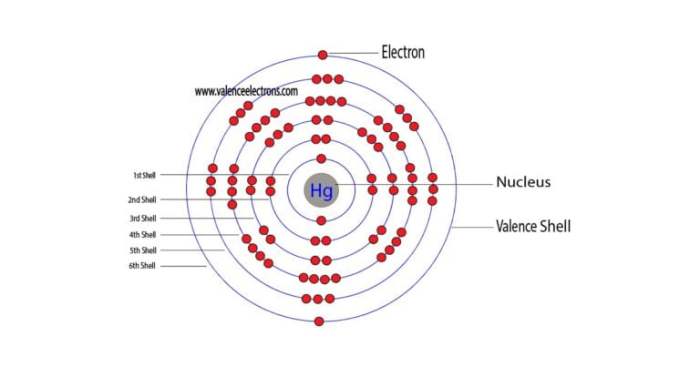
Mercury occupies position 80 in the periodic table, belonging to Group 12 (also known as the Zinc group) and Period 6. Its electronic configuration, according to the Bohr model, is 2-8-18-32-18-2, with two valence electrons in the outermost shell.
This electronic configuration aligns with the trends observed across the periodic table. As we move from left to right across a period, the atomic number increases, leading to an increase in the number of protons and electrons. This results in a gradual increase in the effective nuclear charge experienced by the electrons, causing them to be drawn closer to the nucleus.
Consequently, the atomic radius decreases, and the ionization energy increases.
Similarly, as we move down a group, the number of electron shells increases, resulting in an increase in the atomic radius and a decrease in the ionization energy. This is because the outermost electrons are further away from the nucleus and experience a weaker electrostatic attraction.
The Bohr model successfully captures these trends by depicting the electrons occupying discrete energy levels, with the outermost electrons having the highest energy. The position of mercury in the periodic table and its electronic configuration, as described by the Bohr model, reflect the interplay of these periodic trends, providing insights into the element’s atomic structure and properties.
FAQs
What is the significance of the Bohr model for mercury?
The Bohr model provides a simplified but effective framework for understanding the electronic structure and behavior of mercury, explaining its chemical properties and position in the periodic table.
How does the Bohr model explain the emission and absorption of light by mercury?
According to the Bohr model, electrons can transition between energy levels by absorbing or emitting photons. This explains the characteristic emission and absorption spectra observed in mercury vapor.